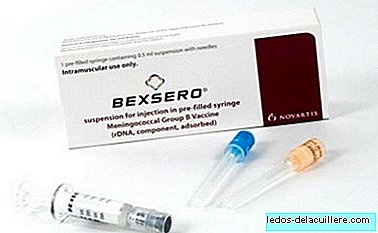
We continue in Spain living a strange situation with the vaccine Bexsero. It is the vaccine that prevents infection that causes meningitis B, and has a still erratic distribution that causes some families to wait for months to immunize their children.
As it has been said for a while this July was when everything was going to be regulated, and it is true that although you still cannot go to the pharmacy and take it to the moment, it seems that more doses are coming (I have administered this week more than in previous weeks).
The fact is that we have new information about this vaccine, specifically that of a recently published study that seems to show that the meningococcal B 'Bexsero' vaccine may be less effective than expected.
Trying to control an outbreak of meningitis in a university
As we read in Healthfinder, the study we talked about was conducted taking advantage of the fact that it occurred an outbreak of meningitis B at Princeton University, in 2013. This outbreak affected nine students and one of them died.
With the intention of controlling it and preventing more students from becoming infected, they decided to administer the Bexsero vaccine to all young people, about 500, being the first time this vaccine was used in the US
Eight weeks after being vaccinated with the two age-appropriate doses, researchers from Princeton University, the University of Minnesota and Public Health England performed blood tests on all vaccinated. They saw that about a third of the students (34%) had not acquired the necessary immunity to consider them well protected. Even so, none of those vaccinated developed a meningitis infection.
In the United States it is not indicated to vaccinate systematically
The vaccine was used for this outbreak because it was learned that it was caused by meningococcus B. However, it is not a disease that occurs often there, and the data from the Centers for Disease Control (CDC) say that Infection rates for this bacterium are in decline since the late 1990s, to the point that in 2013 there were only 550 cases.

In Spain, on the other hand, we have a not much lower number, for a much less numerous population. The Spanish Association of Pediatrics explains that Type B meningococcus is the cause of 7 out of 10 cases of meningitis in Spain and that in the same year 2013 between 400 and 600 affected were registered (the AEP recommends that it be included in the state immunization calendar).
That is why in the United States it is not systematically vaccinated for meningococcus B and it is probably not done at the moment in view of the immunization results, lower than expected. Experts usually indicate that a correct immunization would be one in which 10 or 15 percent of those vaccinated they will be without the necessary antibodies to face a contagion, but in this case it reached 34 percent.
However, Nicole Basta, author of the study, said the following:
The results indicate that we must go deeper to understand the extent of protection that this vaccine could have against the diversity of strains that can cause a meningococcal disease, and especially meningococcal outbreaks.
And although he considers that many students were not well protected, the results could be different with another cut-off point. I explain: the study was based on parameters that are not clear if they are correct. They made a numerical cut that in theory was adequate below which the students did not consider themselves well immunized, however, since there was no contagion and not knowing the vaccine thoroughly, they declared not knowing "what is the point that really means that someone is protected. "
That is why they wanted to be cautious when recommending the vaccine or not and wait for new studies to show what the potency of the vaccine is, what the duration of protection and what the number of antibodies necessary to protect an individual.
So, isn't it a useful vaccine? Is it not effective?
No one has said that. What it seems is that it is less effective than one would expect, at least as seen in this study. Other studies, as we read in Annals of Pediatrics seem to have better data:
The experience in 10 clinical trials in which the immunogenicity of this vaccine was evaluated covers approximately 5,800 people, of which about 4,000 were children aged 2 to 24 months, 84 children aged 40-43 months and 1,738 were adolescents or adults between 11 and 55 years. These studies have shown that the vaccine is immunogenic and safe in all these age groups, and that it induces immunological memory.
But in reality there is nothing clear yet, and that remains to be seen, once the populations are vaccinated, what is the real effectiveness in preventing disease and how long does that protection last. These are data that will be known within a period of time, as conclusions are drawn after the administration of the vaccine in countries such as the United Kingdom, which introduced it in its calendar of funded vaccines as soon as Bexsero was validated for administration.
In the same source, we can also read that Dr. Jerome Kim, from the Seoul International Vaccine Institute in South Korea, explains that "vaccination of all adolescents would prevent 15 to 29 cases and 5 to 9 deaths annual in the United States. " The question that remains then is: and how many would not save?
Photos | iStock
In Babies and more | The meningitis B (Bexsero) vaccine can be purchased in pharmacies on October 1, The AEP asks that the meningitis B vaccine be included in the vaccine schedule, the meningitis B vaccine arrives in Spain but not for all children